All fluids are always evaporating. High temperatures just increase the rate of evaporation.
What is Evaporation ?
A fluid has billions of molecules, all whizzing around at different speeds. Most molecules have a ‘most probable velocity’ (which is low for water at room temperature). Some have low velocities while some have very high velocities.
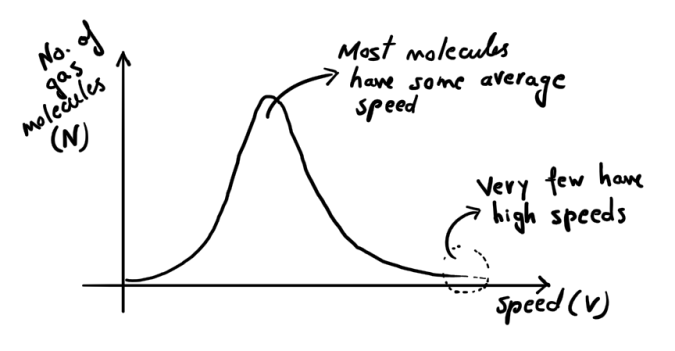
If a molecule at the surface has enough velocity to overcome intermolecular forces of attraction, then it can escape the grip of other molecules. It has evaporated!
As we increase temperature, more and more molecules start achieving a velocity large enough to evaporate. So evaporation rate increases.
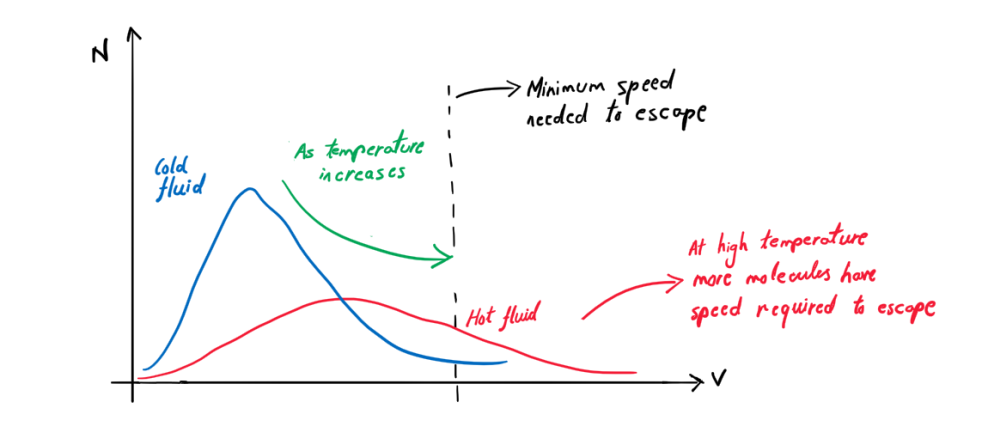
At high temperature, evaporation rate is high. But even at low temperatures, some fraction of molecules will always have enough energy to escape into the air.
There is one last problem. Air can only carry a limited amount of moisture. Evaporating water molecules saturate the air with water so it can’t carry any more. That’s why, water evaporates faster if we blow air over it.
Evaporation is in-fact, the key principle behind cooling devices like AC/ refrigerator. Can you figure out how?
If you liked this article, consider supporting us at Patreon. Become a Patron!
Good Explanation. I liked it.
LikeLiked by 1 person
Thank you. Have you checked any other posts lately ?
LikeLike
In love with these! Couple of questions –
– What determines the most probable velocity of something? Temperature seems to be one factor but what else?
– Is the boiling point a maximum limit on the amount of energy molecules can possess?
LikeLiked by 1 person
Most probable velocity of molecules is mainly determined by the temperature(T) and mass of the molecules(m). Its formula is V = sqrt(RT/m) where R is the gas constant.
Boiling point is certainly not the maximum limit on energy of the molecules. Even at the boiling point, a liquid continues to absorb heat as ‘Latent heat’. So its temperature doesn’t increase but molecules use that energy to break free of intermolecular attraction forces and evaporate. Once all molecules have evaporated, liquid completely turns to vapour. We can continue heating it and now heat will start increasing the temperature of the gas again. This phase is called superheated vapor. Further heating can cause electrons to seperate from the gas molecules and the gas now becomes ionized plasma.
LikeLiked by 1 person
Thanks for the explanation 🙂
LikeLike
Pingback: How will you cool something ? | Yellow belt blog
Pingback: Physics behind water in space | Yellow belt blog
Very NIce. Keep it up.
LikeLike